
For plant cells, which are often highly pigmented, this represents a loss of information.
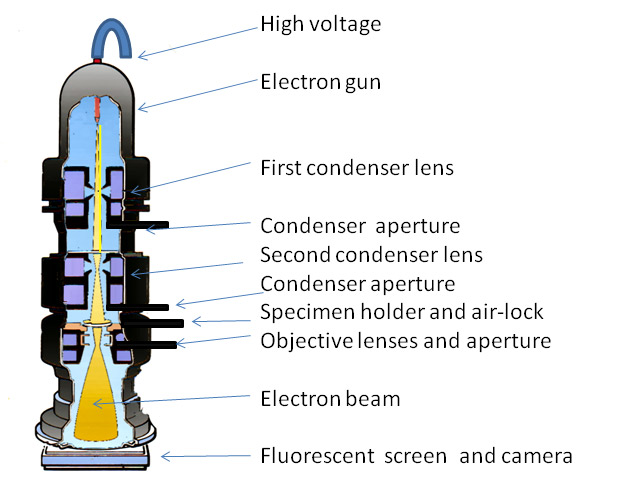
Moreover, as these detectors do not detect the wavelength of the incident light, information about the wavelengths of light being absorbed by the sample is not recovered by the conventional transmitted light detector. Various forms of transmitted light microscopy can be achieved in association with confocal microscopy, including brightfield, polarised light, phase contrast and differential interference contrast. The image is non-confocal and contains out-of-focus light as it is formed by light that does not pass through a pinhole, but is does provide context for confocal fluorescence observations. Thus, focus adjustments and modifications to the settings of these diaphragms will affect image formation. An insertable mirror then reflects the transmitted light to the detector mounted in a conjugate plane to the lamp filament. The optics of the transmitted light detector requires that the light passing through the sample to be collected by the condenser lens (rather than an objective lens), and focussed through the condenser and field irises, travelling in the reverse direction to normal, transmitted light illumination. As a consequence of this, these transmitted light detector images can be combined with the fluorescence images into overlays in ways not possible with photographs taken with an external camera.
TRANSMISSION LIGHT MICROSCOPY REGISTRATION
Importantly, images detected by the transmitted light detector are recorded concurrently with confocal images meaning that they have both spatial and temporal registration with the confocal images. In this configuration, the light transmitted through the sample can also be collected with the condenser lens and imaged with a transmitted light detector, and most confocal microscopes are now equipped with transmitted light detectors. These fluorescence techniques typically use microscopes in the epifluorescence configuration in which the excitation and emission light is delivered and collected through the same lens. GFP expression has been visualised with advances in microscopy including the advent on confocal and two photon microscopy and the more recent arrival of super-resolution imaging and light sheet microscopy. Recent decades have seen a revolution in the imaging of living plant cells, with the expression of green fluorescent protein targeted to almost all locations within the plant cell through different protocols including transient particle bombardment, virus-mediated infection and stable Agrobacterium-based transformations. This has been documented in red onion epidermal cells where changes in vacuolar pH triggered by the weak base methylamine result in measurable colour changes in the vacuolar anthocyanin. Changes in sample colour can be quantified by transmitted light imaging. If the blue light used for YFP excitation is blocked from the transmitted light detector with a cheap, coloured glass filters, the non-absorbed green light will form an improved transmitted light image. Transmitted light images of Arabidopsis leaves expressing GFP can be improved by concurrent illumination with green and blue light. For faster imaging of pigmented samples, transmitted light images can be formed with non-absorbed wavelengths. The resulting transmitted light images can be optimised and merged in ImageJ to generate colour images that maintain registration with concurrent fluorescence images. Pigmented samples can be imaged in real colour using sequential scanning with red, green and blue lasers. Because plants often provide difficulties for taking transmitted light images, with the presence of pigments and air pockets in leaves, this study documents several approaches to improving transmitted light images beginning with ensuring that the light paths through the microscope are correctly aligned (Köhler illumination).


 0 kommentar(er)
0 kommentar(er)
